“Loop mediated isothermal amplification is a versitile technique and without a doubt one of the most important tools for high-quality DNA analysis outside of specialized laboratories.”
Loop mediated isothermal Amplification (LAMP) & Lateral Flow
Loop mediated isothermal Amplification – Introduction
If you are confronted with the topic of “isothermal DNA amplification” for the first time, the chances are very high that a method called LAMP is somehow involved. This has nothing to do with light bulbs or LEDs. LAMP stands for Loop mediated isothermal amplification, a DNA amplification method that is usually carried out at temperatures between 60 and 70°C. LAMP is particularly characterized by its excellent sensitivity, amplification effiency and high reaction speed.
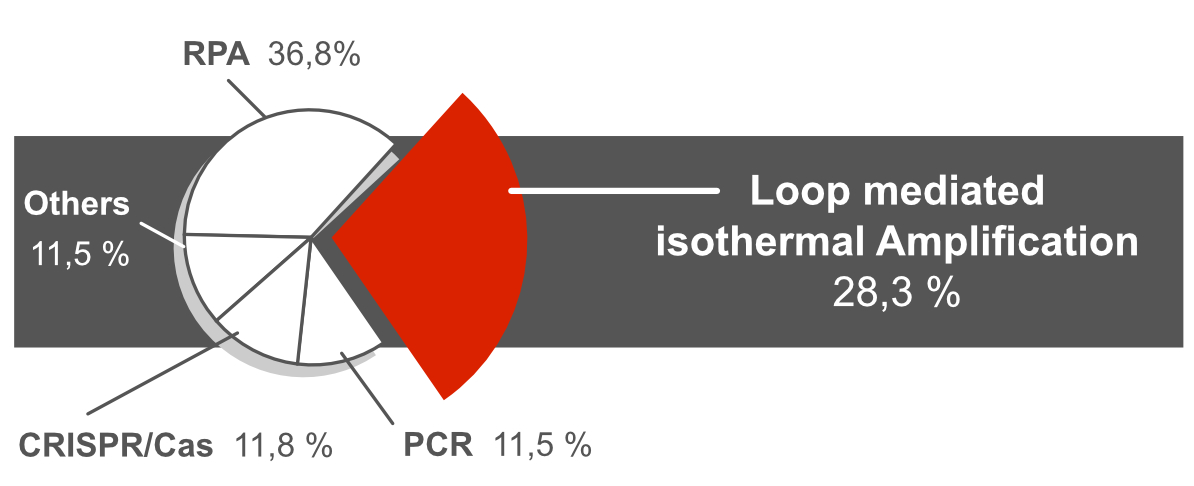
A literature study containing more than 300 peer reviewed publications revealed that LAMP and RPA are the most frequently used DNA amplification methods combined with a rapid Lateral Flow readout. Approx. 28% of these publications used the LAMP method for nucleic acid amplification.
History of the Loop mediated isothermal Amplification
Inspired by existing isothermal DNA amplification methods, Notomi and colleagues initially described the loop mediated isothermal amplification method for the first time in 2000. They used existing knowledge and invented an amplification method that is highly efficient without the need for addtional enzmyes beside a DNA-Polymerase. The creation of a reaction intermediate, which has terminal, single-stranded loop structures, allows multiple annealing and elongation options. As a result, the reaction is extremely efficient and reaction products of various size are amplified. At that time, the authors were able to conclude that the (RT) LAMP works fast and efficient with a sensitivity comparable to that of PCR. It was pointed out that LAMP reaction products can be easily and quickly detected via antigen-antibody-interaction. From today’s perspective, this can already be understood as an indication for the potential of the combination of loop mediated isothermal amplification and Lateral Flow. One of the first papers on this topic appeared in 2008. An RT-LAMP reaction product was specifically detected with the universal Lateral Flow Device, Milenia HybriDetect. The specific and sensitive detection of a viral shrimp pathogen was achieved in about an hour. Since then, this technology has been continuously developed, adapted and optimized, so that today the LAMP represents one of the most promising tools for a new generation of molecular Point-of-Care diagnostics.
Mechanism
The name “Loop mediated isothermal Amplification” is a very apt description of the molecular amplification mechanism. In general, LAMP is more complex than the other amplification methods, such as PCR or RPA. The use of 4 to 6 primers in combination with a DNA polymerase with lacking exonuclease activity and strong strand displacement activity is the basis for an isothermal LAMP reaction. The following figure shows the complex primer-/ probe-arrangement which is neccessary for a well working LAMP assay.
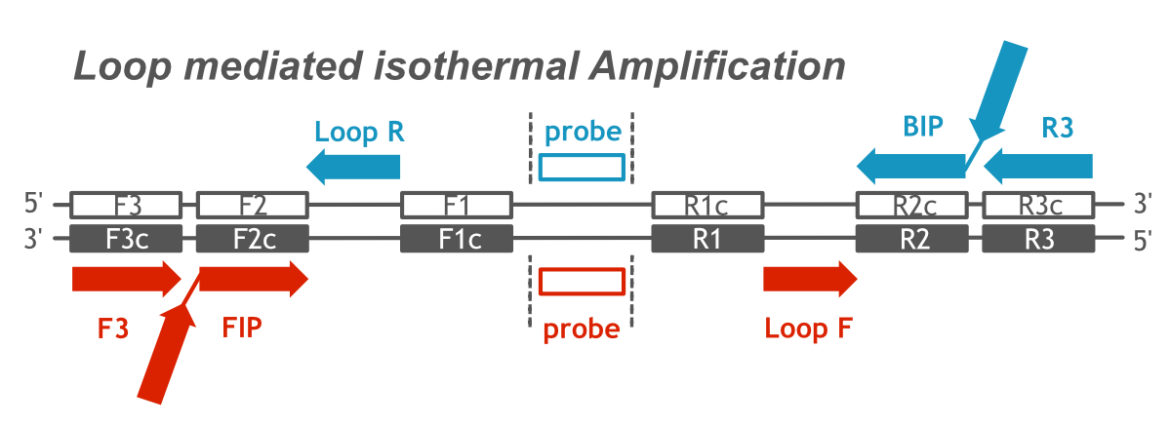
Complex Primer-/ Probe-Arrangement of LAMP assay
Characteristic LAMP incubation temperatures are between 60 and 70°C, which allows initial primer invasion into weakened doublestranded DNA (template). This can be improved in some cases by reaction additives such as betaine. In the initial phase of amplification, a dumbbell-shaped reaction intermediate is formed due to the elongation of large modular primers and continuous strand displacement of primary amplicons. The terminal loops of this intermediate structure are the starting point for the exponential DNA amplification. In contrast to other DNA amplification methods, significantly more product is amplified during LAMP containing amplicons of many different sizes. The following figure is explaining the amplification mechanism in more detail.
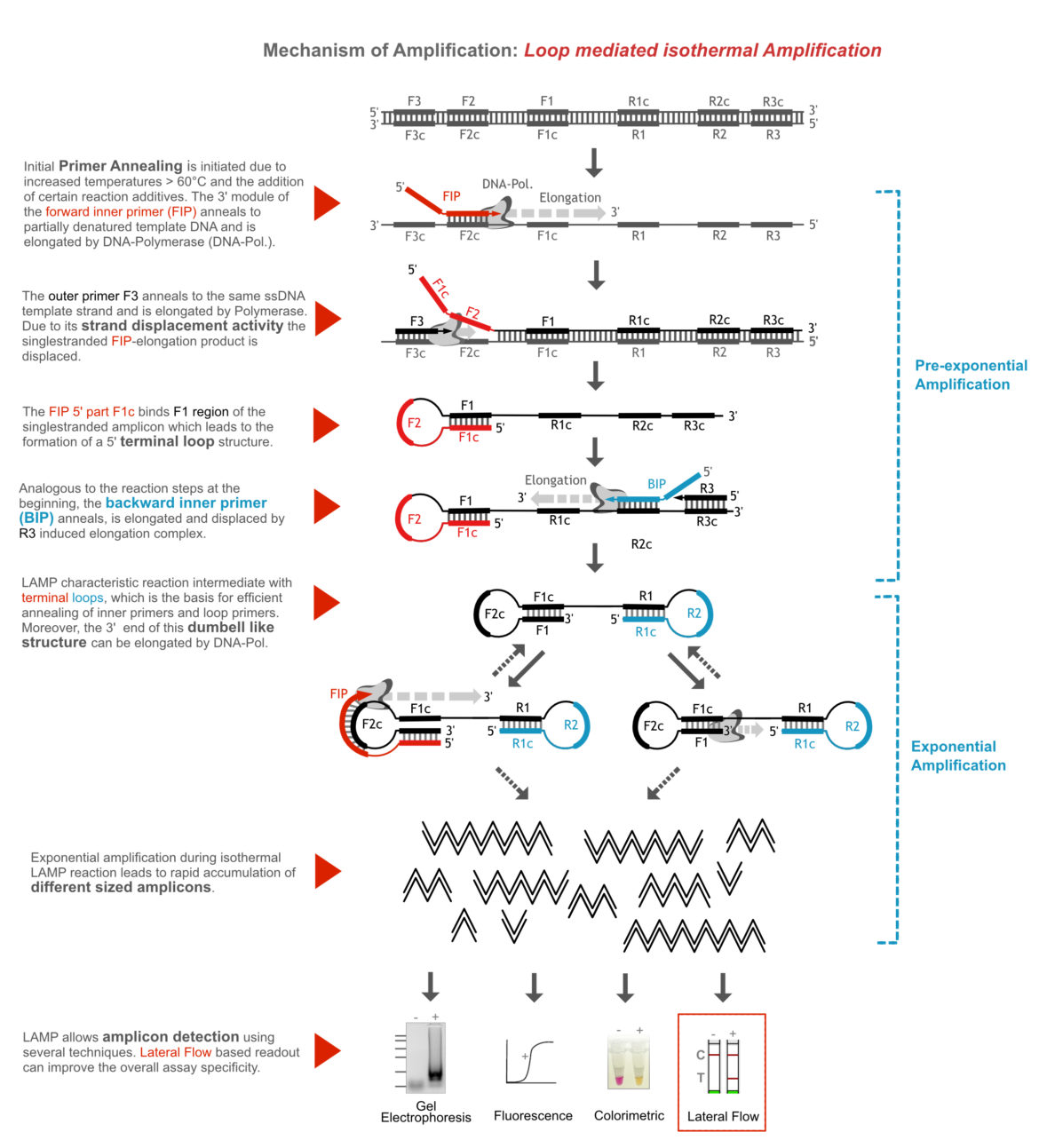
How to Modify my LAMP Assay for Lateral Flow Readout
LAMP requires a relatively complex primer design. 4 to 6 primers are necessary to initiate efficient DNA amplification. Due to the complexity, it is not recommended to design LAMP-Primers by hand. Rather, it makes sense to use available online tools to find an appropriate primer set and use this as a basis for further optimization. In order to visualize LAMP reaction products on a Lateral Flow Device, it is recommended to use a labeling strategy which allows incorporation of Biotin- and FAM/FITC-lables into amplicons. The easiest way to integrate neccessary labels is the usage 5‘ labeled LAMP primers. Therefore, it can be beneficial to test various labeling options (e.g. FIP & LF, FIP & BIP, LF & LB, etc.). Moreover it is possible to intergrate an additional probe hybridization, in order to improve overall assay specificity.
LAMP & Multiplexing
Despite the complex primer design, LAMP is a promising tool for the development of multiplex DNA amplification assays. Once a suitable primer combination has been found, duplex or triplex amplifications can also work with satisfying accuracy and sensitivity under low resource settings. The hybridization of specific probes to amplicons is a frequently used methodical tool in LAMP applications in particular, which of course can also be an important facet for increased multiplexing in the context of lateral flow. We at Milenia Biotec see the enormous potential of multiplex LAMP lateral flow assays. The future will show whether this actually proves to be true.
Limitations
The LAMP is a versatile tool and offers numerous possibilities, especially in combination with lateral flow. Like any other method, this technique has advantages, but also disadvantages. An important LAMP-characteristic limitation is the complex assay design. Of course, there are very good, available LAMP primer design tools, such as NEB‘s Primer Design Tool or the Primer Explorer (V5) from Eiken. When using less suitable sequence sections or multiplexing is neccessary, the development is often complicated. In such cases, significantly more energy, money and time has to be invested in the development compared to other DNA amplification methods. The induction of the so-called high dose hook effect or the problem of carryover contaminations are LAMP-LFA characteristic pitfalls. But both phenomena are more connected to handing mistakes, rather than methodological limitations.
Benefits
A LAMP reaction is able to detect low copy DNA targets in less than 30 minutes. In some publications amplification speed could be further accelerated. The time and energy invested in assay design pays off. Once a suitable primer combination has been found, the LAMP works with excellent sensitivity, which is often comparable to established RT-PCR applications. Very sensitive DNA analysis methods are often a blessing and a curse at the same time. Carryover contamination is a common phenomenon, especially for nucleic acid lateral flow assays. The very good compatibility of the LAMP reaction with the uracil-DNA-glycosylase (UDG / UNG) for prevention of carryover contamination has been shown several times and thus has to be highlighted as an important advantage of this method.
| Method combined with LFA | Sensitivity | Specificity | Speed | reverse Transcription (one pot) | Robustness | Method. Variety | Simple Assay Design | Multiplexing Ability | Prevention Carryover Cont. |
|---|---|---|---|---|---|---|---|---|---|
| PCR | |||||||||
| LAMP | |||||||||
| RPA | |||||||||
| CRISPR (Label-Sep.) | |||||||||
| CRISPR (Label-Inc.) |