
Shortly after the publication, I had the chance to visit the authors Niall Armes and Olaf Piepenburg in Cambridge, UK and it was a meeting that I will never forget! Niall and Olaf were very enthusiastic about their idea of the „recombinase polymerase amplification“, called RPA, and they gave me a very detailed and inspiring presentation about their new technology. They had founded the company ASM Scientific Ltd., which was later renamed in TWIST DX. They had the plan to let the world know about their new development. They were very proud that Cary Mullis, inventor of the PCR, sent them a message with his congratulations on the development of their isothermal RPA.
Later TWIST DX started to offer complete kits for the development of RPA tests. One of these kits, the TWIST amp nfo kit, required lateral flow strips for the detection of the RPA amplicons. Since then, the Milenia HybriDetect kits (MGHD 1 and MGHD2 1 – see table 1) were offered in combination with the TWIST DXkits.
| Product Name | Milenia HybriDetect | Milenia HybriDetect 2T |
|---|---|---|
| Order Code | MGHD 1 | MGHD2 1 |
| Number of tests | 100 dipsticks | 100 dipsticks |
| Number of test lines (T) | 1 | 2 |
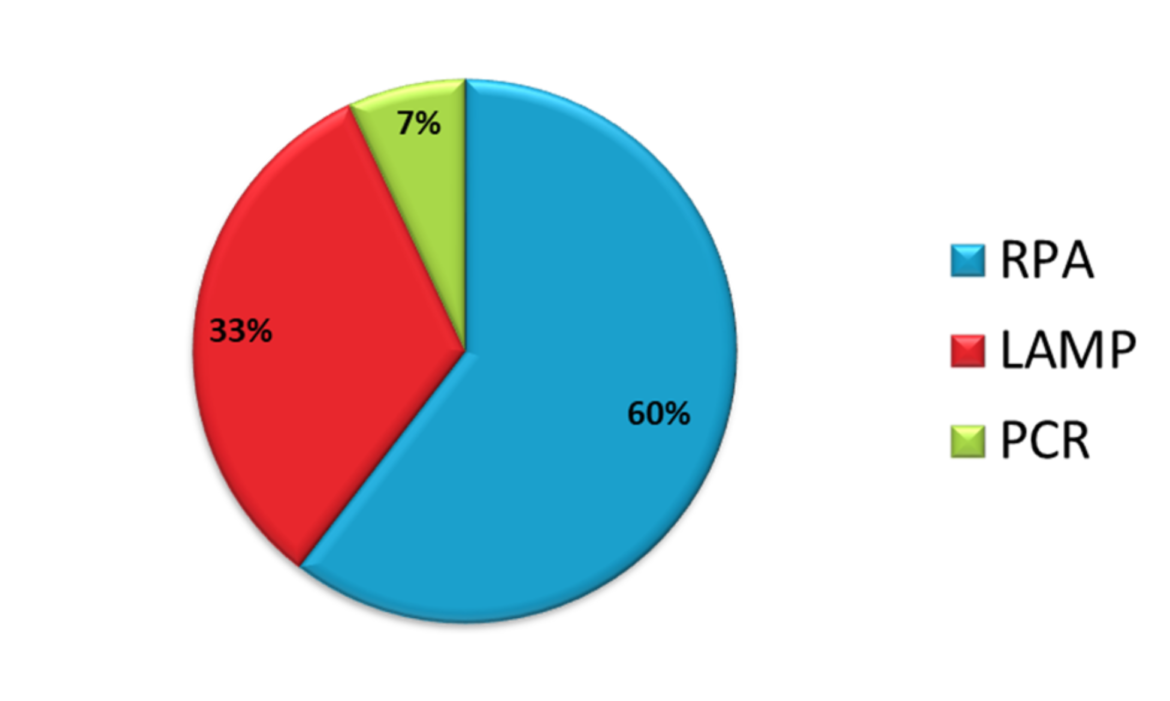
79 publications of the HybriDetect Citation List are RPA based
After TWIST DX has launched the TWIST amp nfo kit, a lot of researchers have done high quality studies with these reagents. Therefore, it is no surprise that 79 of the 129 publications about the use of Milenia HybriDetect are related to the use of RPA. Another 42 papers are referred to the use of the LAMP (loop-mediated-isothermal-amplification) method, another isothermal amplification method and 9 papers are referring to PCR applications – see Figure 1.
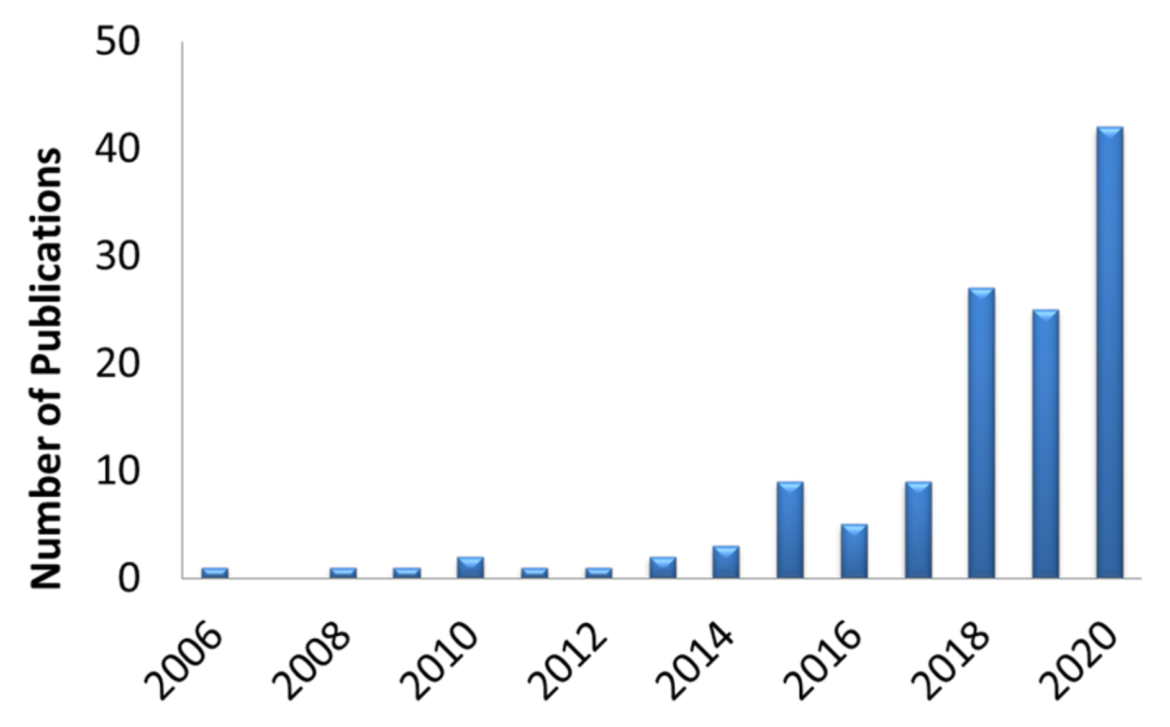
Furthermore, as shown in Figure 2, the annual number of publications citing the use of Milenia HybriDetect is rapidly growing. In the year 2006 Piepenburg et al. was the only group, referring to the use of Milenia HybriDetect. In the first 6 months of 2020 altogether 42 scientific papers on the use of Milenia HybriDetect have been published so far. This trend is based on the increasing awareness researchers have, regarding the potential of the product in their research work. The trend also shows that the development of new methods and clinical applications can benefit from the features of Milenia HybriDetect. including gene editing technologies, like CRISPR. These new technologies open up the chance to create easy to use tests, especially for the detection of viruses, like Ebola-, Zika-Virus and SARS-CoV-2, with an outstanding sensitivity.
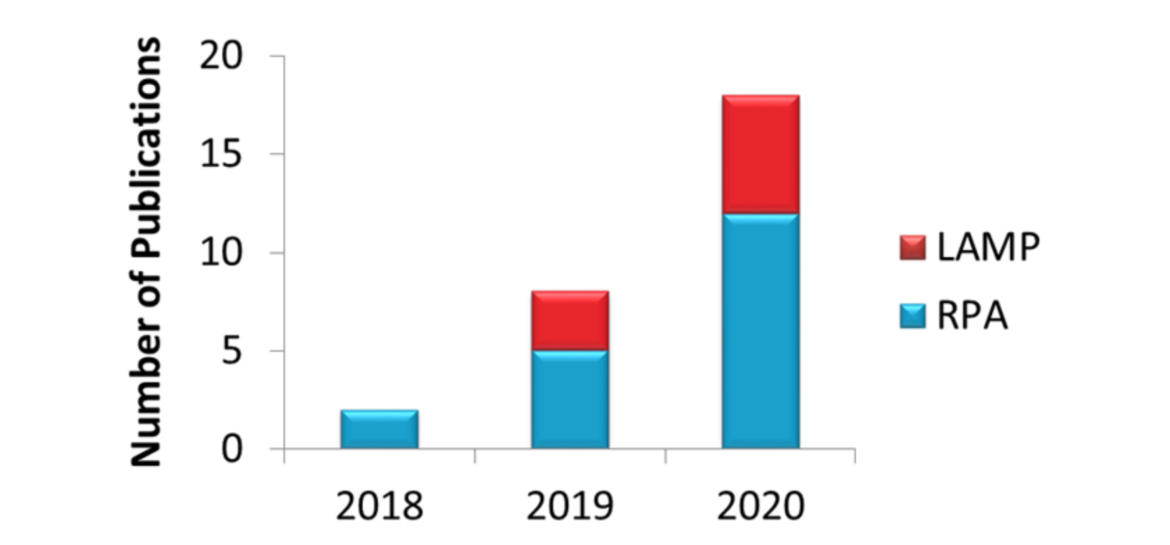
Combining Gene Editing Technologies, including CRISPR applications with Milenia HybriDetect
In the year 2018 my attention was drawn to the first CRISPR based application which was combined with the use of Milenia HybriDetect.
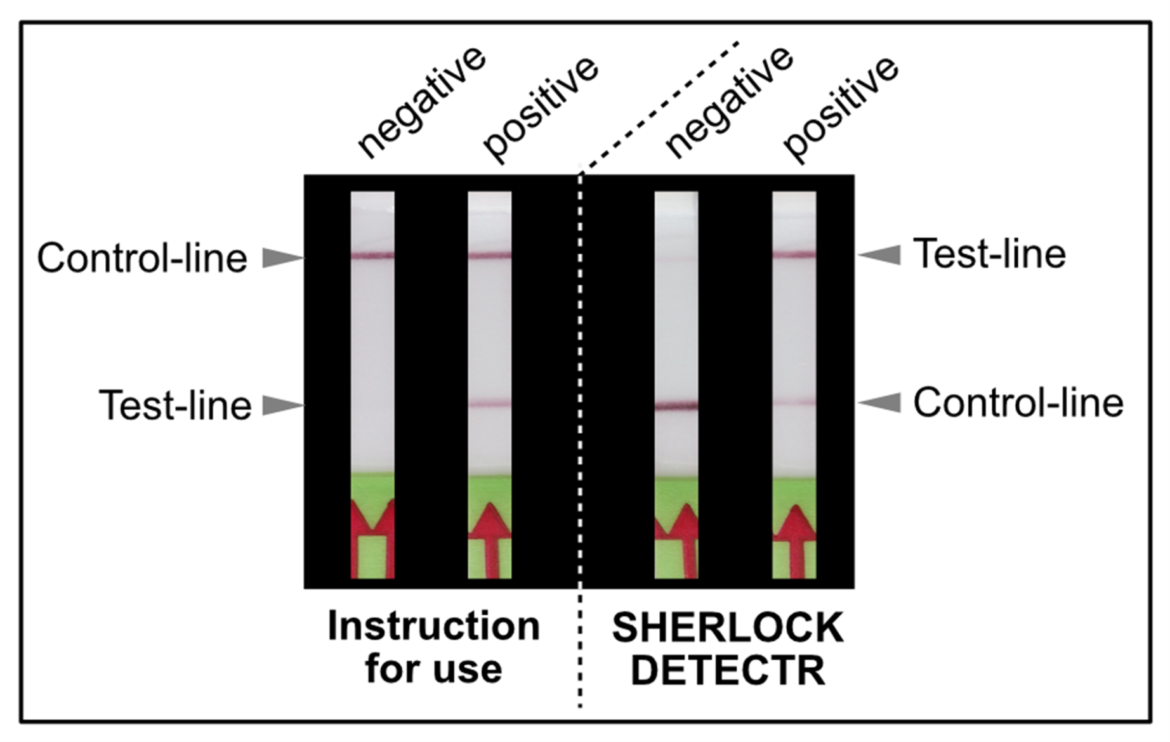
In CRISPR methods, a reporter is cleaved by CRISPR/Cas upon activation by the presence of a specific target gene. For CRISPR applications with the detection via Milenia HybriDetect, the reporter is labeled with biotin on the one end and a FITC or FAM on the other end. In case the sample is negative, the reporter binds at the bottom line of the HybriDetect test strip and retains all gold particles coated with an anti-FITC antibody. For this reason the top line on the strip will not be colored. If the sample is positive, the reporter will be cleaved and consequently gold particles will continue to travel upstream across the test strip and the top line of the strip will show up due to the binding of gold particles. This also means that in CRISPR applications the meaning of T- (test) line and C- (control) line is changed. In CRISPR applications the top line is the T-line and the bottom line is the C-line, while in amplification tests it is vice versa. (Figure 3). This and other aspects of CRISPR applications have been discussed in our blog previously. For more Information see the blog articles: Lateral Flow Readout for CRISPR/Cas-based detection strategies and Diagnostic CRISPR Tools and Techniques.
Milenia HybriDetect and SARS-CoV-2
13 of the 42 papers published in 2020 citing the use of Milenia HybriDetect are referring to the detection of SARS-CoV-2. At the end of 2019 the outbreak of COVID-19 started in Wuhan, China and spread out over the entire world within the year 2020. Many creative scientists have developed powerful tools for the detection of SARS-CoV-2. Some of these tests need only minimal equipment, are easy to handle, allow reporting results in less than one hour, are extremely sensitive and can possibly be done at the point of care! 13 of the 42 scientific papers published in 2020 so far refer to the use of Milenia HybriDetect on SARS-CoV-2 detection and allow a near patient application of these tests. Especially in the US some startup companies, including SHERLOCK Bioscience, Mammoth Bioscience and CASPR Biotech, have been founded to develop and manufacture easy CRISPR based methods to detect SARS-CoV-2 and are in a phase of FDA submission of these tests.
There is an exciting time to come – Keeping the Milenia HybriDetect Citation List updated
Since many years we keep the Milenia HybriDetect Citation List updated every 6 months. It is always exciting to search for new high quality publications citing the use of our products and we strongly believe that a lot more papers will be published in the next few years.
If you like to get the updates on a regular basis or you can contribute a paper to the list, please send us an e-mail to: info@milenia-biotec.de.
Thank you very much in advance to get in touch with us! We are really looking forward to hear from you!
If you would like to know more about our products and news about Milenia Biotec, please follow us on LinkedIn.

